Biobanking
INLANDYS® automated platform integrates with existing tanks and LIMS, for any size of biorepository
- Guarantees user safety
- Ensures all sample traceability
- Maintains sample integrity
- Improves biobank management efficiency
- Guarantees user safety
- Ensures all sample traceability
- Maintains sample integrity
- Improves biobank management efficiency

Biobanking
INLANDYS® automated platform integrates with existing tanks and LIMS, for any size of biorepository
- Guarantees user safety
- Ensures all sample traceability
- Maintains sample integrity
- Improves biobank management efficiency
- Guarantees user safety
- Ensures all sample traceability
- Maintains sample integrity
- Improves biobank management efficiency

As the number of stored samples continuously grows, and thus the number of manual retrieval and storage operations, automation of cryogenic biobanks is a key opportunity to improve both sample quality and user safety.
Automation that matches with existing equipment and practices
IRELEC has designed, INLANDYS®, a full automated system with a unique architecture, that fits with existing biobank equipment and database software:
-
The system is built with a standard storage architecture: samples are stored in boxes stowed inside racks in cryogenic tanks
-
It works with the existing tanks, by just adding an automation kit
-
It is linked to the biorepository database software
Best user safety and working conditions
All operations are programmed by the user on its database software.
INLANDYS® carries out all painstaking operations: tank opening, rack retrieval from tank, box retrieval from rack, samples transfer.
A shuttle box (empty or with ready-to-be-stored samples) is placed by the user on a secure transfer drawer which provides the access to the secure automated system: closed area with safety PLC.
Efficient and Easy-to-operate
INLANDYS® greatly improves user experience: just one single scheduling is necessary for the retrieval or storage of up to hundreds of samples, and the user is not required to wait during operation. He or she is informed when the programmed operation has been completed.
A seamless access to on-going operation status is provided.
Cold chain management and traceability of samples
A perfect command of the cold chain is one of the key success factors of this system: As sample exposition time to room temperature is critical, transfer operations are done in nitrogen-cooled areas. Each sample is individually monitored, during its entire storage life, from its initial deposit to its final delivery, during all of its movements outside the cryogenic tank due to the retrieval or the storage of neighbouring samples.
Sample identity is monitored with cameras unit-by-unit.
White Paper - Cryogenic Bio-storage Management
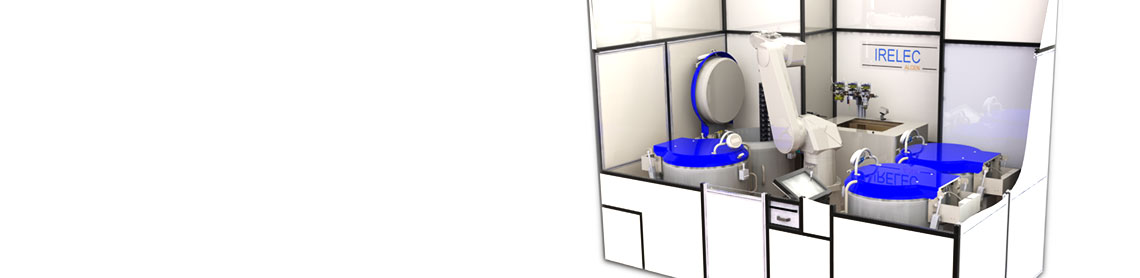
Automated Compact System
Stand-alone automated platform
- On the shelf technology
- 1 to Up to 4 cryogenic tanks
Automated Compact System
Stand-alone automated platform
- On the shelf technology
- 1 to Up to 4 cryogenic tanks
Automated Extensible System
Custom automated platform
- Adapted to specific environment and constraints
- Unlimited number of tanks
Automated Extensible System
Custom automated platform
- Adapted to specific environment and constraints
- Unlimited number of tanks
References
| The Biological Resource Centre of Grenoble University Hospital is equipped with an INLANDYS® compact system |
|---|